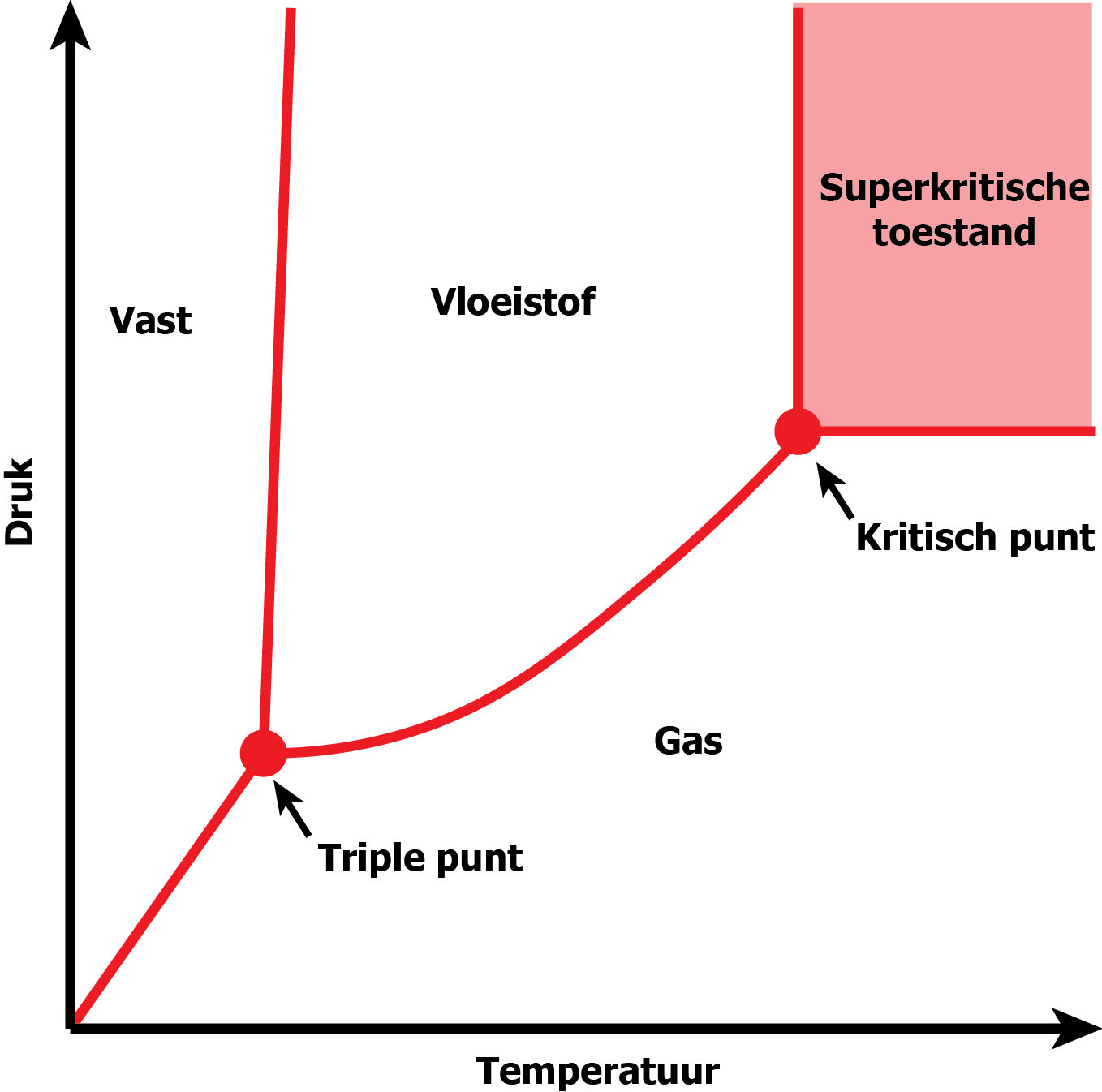
What is supercritical CO2
?
Compared to conventional solvents (e.g. hexane), supercritical CO2 has the advantage of being considered a green solvent. Carbon dioxide is considered safe because it is non-flammable and has a high chemical inertness. It is a relatively inexpensive product that is also easy to recycle. It is also ideally suited for extraction of temperature- and oxidation-sensitive substances.
What is supercritical CO2 extraction?
Supercritical CO2-extraction is the process of using supercritical CO2 to extract certain substances. This is done using a purpose-built extractor.
1 The central part of the
extractor is the extraction vessel. It contains the material from which one wants to extract.
2 The extraction vessel is located in an oven or is heated directly to the correct temperature.
3 The
pump
brings the gas from the CO2 storage to the extraction vessel and the pressure regulator (on the back of the extraction vessel) is used to place the CO2 at the
correct pressure.
4 Once the temperature and pressure in the extraction vessel are high enough, the CO2 will be in a
supercritical state and the
extract can be absorbed by the CO2.
5 The CO2 with the extract leaves the extraction vessel at the other end. After the pressure regulator, the pressure of CO2 is lowered causing it to go back into gas state.
6 The extract is released and left behind in a collection tank. After extraction, the CO2 gas can be captured for reuse.
Equipment
The platform currently has two installations in operation.
The combination of these two devices makes it possible to develop upscaling models.
In addition to these two devices for extraction, the platform has the necessary techniques and equipment for sample preparation and analysis of the extracts.